World-first immunotherapy trial to treat Type 1 diabetes
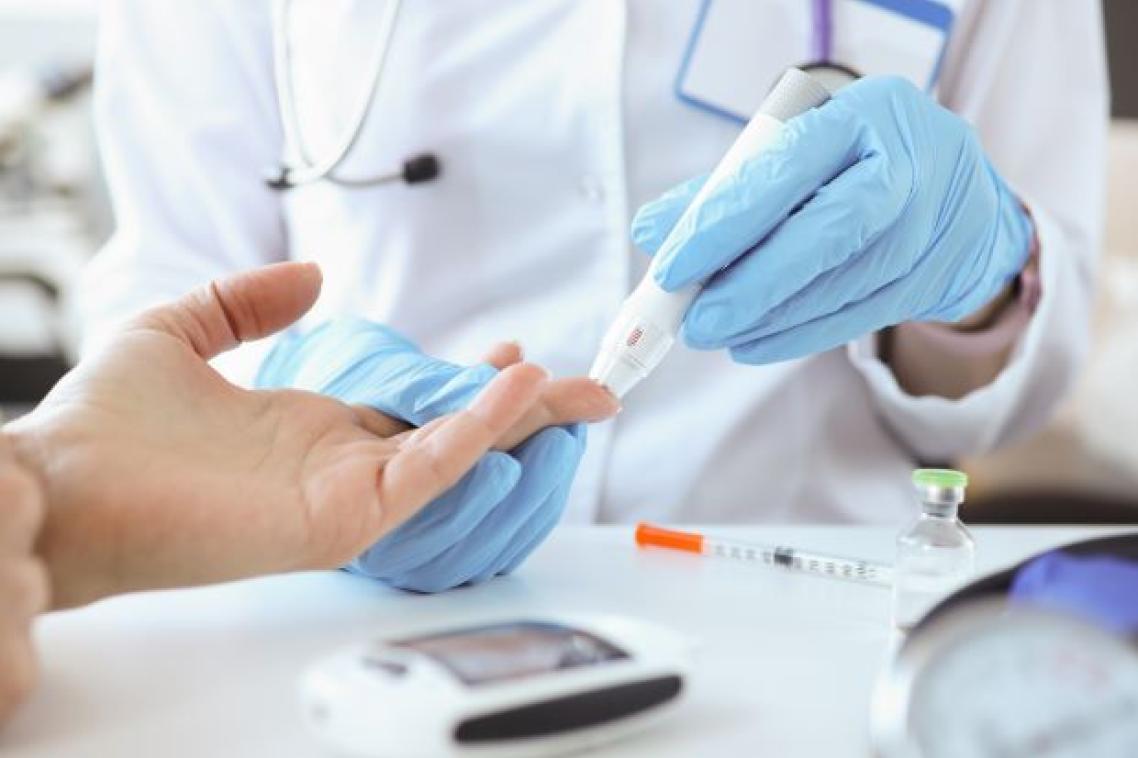
Researchers at The University of Queensland have dosed 5 participants in the first clinical trial of a potentially revolutionary immunotherapy drug to treat Type 1 diabetes.
Professor Ranjeny Thomas AM from UQ’s Frazer Institute has led the development of a targeted immunotherapy drug, ASITI-201, which has been designed to rebalance the body’s immune response to protect insulin-producing pancreatic cells.
“In people with Type 1 diabetes, the immune system starts to recognise pancreatic cells as something it needs to attack, and right now the only available treatment is insulin replacement,” Professor Thomas said.
“We’ve taken a new approach and developed ASITI-201 using a protein from the pancreas, along with vitamin D to calm the immune response.
“This potential treatment uses the immune system’s ability to heal and has been successful in controlling the disease in mice.”
Lead investigator Dr Aakansha Zala said the drug candidate aims to preserve as much pancreatic function for as long as possible in people recently diagnosed with Type 1 diabetes, to reduce the amount of insulin they need to administer.
“We are looking for people over the age of 18 who have been diagnosed with Type 1 diabetes within the last 5 years, to participate in the trial at the Translational Research Institute’s Clinical Research Facility based at Brisbane’s Princess Alexandra Hospital,” Dr Zala said.
“We will be looking at whether the drug changes the immune system in the way that we anticipate.”
Type 1 diabetes affects more than 120,000 Australians and usually develops in children and young adults.
“Ultimately, we need to move to larger trials which include children, who have a much faster progression to insulin dependence,” Professor Thomas said.
“We've been developing a similar drug with the potential to treat Rheumatoid Arthritis, and that knowledge has supported our development of this drug for Type 1 diabetes.”
The trial itself is being funded by the Medical Research Future Fund via Australia’s national biotech incubator CUREator which helped form spin-out company Liperate Therapeutics – which was established by UQ’s commercialisation company UniQuest.
The drug’s preclinical proof of concept was supported by multiple grants from Breakthrough T1D (formallyJDRF) totalling $2.54 million between 2003-2015.
The Leona M. and Harry B. Helmsley Charitable Trust subsequently awarded two grants totalling $5.33 million to complete the preclinical development of the drug candidate, including safety studies and manufacturing.
Dr Ben Williams, Program Officer at Helmsley Charitable Trust, said the organisation is proud to have supported the research which is a step towards improving the lives of people with Type 1 diabetes.
“Bringing drug candidates from the laboratory into clinical testing is always a herculean effort,” Dr Williams said.
The Helmsley Charitable Trust is the largest private funder dedicated to transforming the trajectory of Type 1 diabetes.
Topics
Related articles
Brisbane woman runs 50km for 50 days to break barriers for para-athletes